Effervescence is the escape of gas from an aqueous solution. The term is used to describe the foaming or fizzing that results from a release of gas. In the lab, a common example of effervescence is the addition of hydrochloric acid to a block of limestone. If a few pieces of marble or an antacid tablet are put in hydrochloric acid in a test tube fitted with a cork, effervescence of carbon dioxide can be witnessed.
This process is generally represented by the following reaction, where a pressurized dilute solution of carbonic acid in water releases gaseous carbon dioxide at decompression:
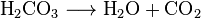
In simple terms, it is the result of the chemical reaction occurring in the liquid which produces a gaseous product.







No comments:
Post a Comment